Atlanta 3-year-old rolls up sleeve to test COVID-19 vaccine
3-year-old Atlanta girl joins Pfizer vaccine study
U.S. officials say younger children could start being vaccinated as soon as this fall and winter once pediatric vaccine trials end.
ATLANTA - Three-year-old Astrid Pearson did not seem to be sweating her first shot appointment at the Emory Vaccine Research Center Wednesday.
She sat on an exam table surrounded by her toys.
Her mother Dre Pearson says she had tried to prepare her daughter.
"I explained to her she was going to get a blood draw and her nose tickled, and she was going to get a shot, but it's a very special shot," Pearson says.
Special, because Astrid Pearson is enrolled in a pediatric vaccine trial of the Pfizer-BioNTech COVID-19 vaccine.
The Emory team eased her through her first dose of the test vaccine with juice and stickers, while Astrid played games on her mother's cellphone.
"They were, like, 'After this blood draw, we have a treasure box, and then you can get this sticker, and then another sticker,’" her mother laughs.

Three-year-old Astrid Pearson is part of a Pfizer-BioNTech pediatric vaccine trial at Emory Vaccine Research Center in Atlanta. (Dre Pearson)
Astrid, who is from Ormewood Park in East Atlanta, is in the 3-to 5-year-old volunteer group, one of about 4,600 children trial organizers hope to enroll in this study across the US.
This is a dosing trial, designed to find out which vaccine dose is safest for younger kids, and whether that dose can produce an effective immune response in children from the ages of 6 months to 11.
The children are divided into 3 groups, those ages 5 to 11, 2 to 5 and 2 to 6 months.
Her mom was a volunteer in another vaccine trial.
"At the end of the day, I feel confident in the process, to the point that I'm also willing to have my daughter participate in a trial," Pearson says.
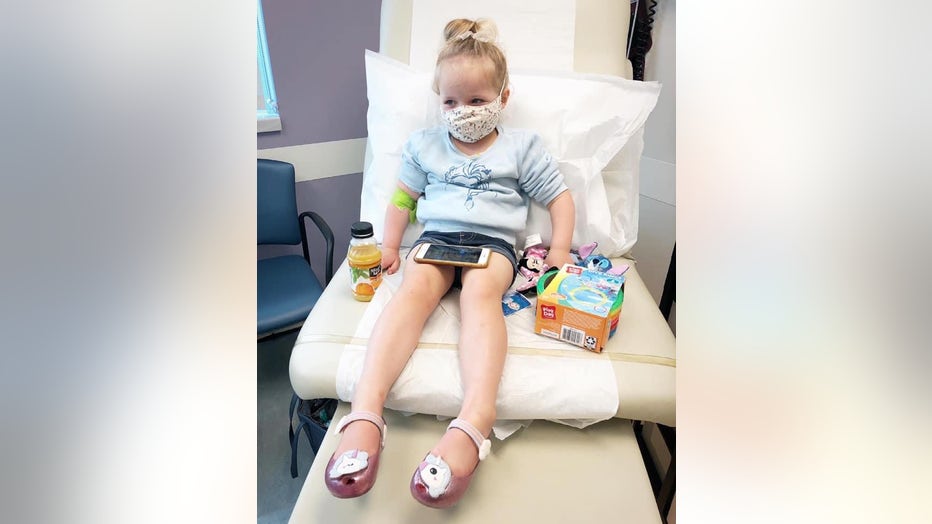
Three-year-old Astrid Pearson waits to receive her first dose of the Pfizer-BioNTech COVID-19 vaccine at Emory Vaccine Research Center in Atlanta.
Pearson says she has heard the reports younger children typically don't get very sick, if they get ill at all, with COVID-19 infections.
She knows some parents question whether vaccinating children is even necessary.
"We, actually, unfortunately, have a couple of friends whose child has gotten sick, and they've gotten quite sick," Pearson says. "So, I would like to save my child that discomfort."
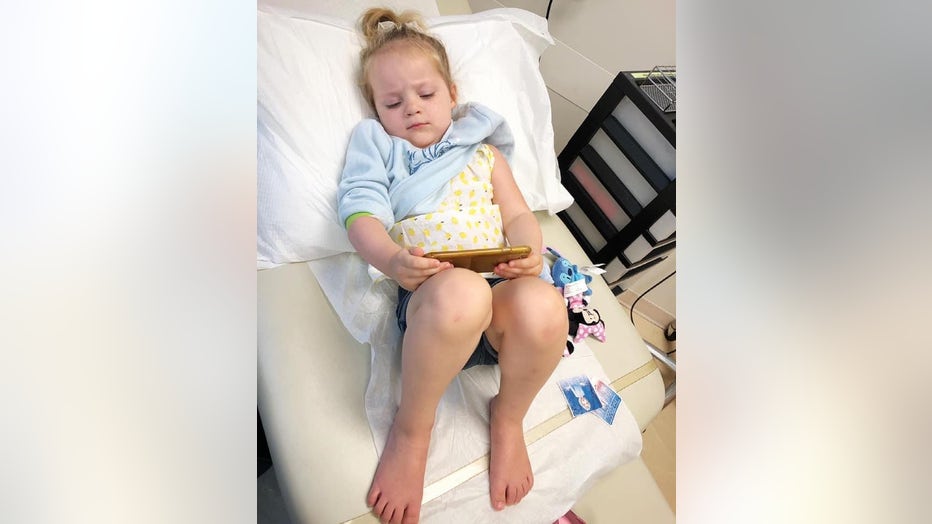
Three-year-old Astrid Pearson of Atlanta plays on her mom's cellphone after receiving a shot at the Emory Vaccine Research Center in Atlanta.
As part of the study, she is keeping an electronic diary of Astrid's symptoms after each dose of the test vaccine.
"Last night I filled out that her arm had a little bit of swelling and that she had a little soreness," Pearson says. "Today, I will mention the fatigue and probably leave another note about how she didn't sleep last night, because I suspect she might be a little achy."
A day after her first dose, Pearson’s daughter is back in her daycare pod, although her mom says she does seem sleeping.
She is also really taken with the bandage she was given after her blood draw.
"She was showing it off all day yesterday," Pearson says. "She refused to take it off. She said, 'I got a special bandage, because I got a special shot.’"
The Pearsons will not know for 6 months whether Astrid is in the group that received the test vaccine or the placebo.

Three-year-old Astrid Pearson poses with her mother Dre Pearson in Atlanta.
She will receive her second dose in three weeks.
All of the participants will have the opportunity to receive the vaccine during the trial, which will run for two years.
WATCH: FOX 5 Atlanta live news coverage
_____
Sign up for FOX 5 email alerts
Download the FOX 5 Atlanta app for breaking news and weather alerts.

