Emory enrolling seniors for possible coronavirus vaccine tests
ATLANTA - Emory University has begun enrolling Georgians who are older than 55 years old for a clinical trial of a possible vaccine for the coronavirus.
App users click here for live updates
The testing of this new potential vaccine began on March 16 and involved 45 health volunteers between the age of 18 and 55 at Emory and the Kaiser Permanente Washington Health Research Institute in Seattle. An additional trial site at The National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC) clinic in Bethesda, Maryland has been added as well.
The "Phase 1" trial is supported by NIAID, part of the National Institutes of Health.
Sign up for FOX 5 email alerts
After testing, investigators are now expanding the trial to 30 people between the age of 56 and 71 and 30 people who are older than 71.
Health officials say enrolling older volunteers will help investigators understand what vaccination could do in older Georgians, who face higher complication rates from the virus compared to younger people.
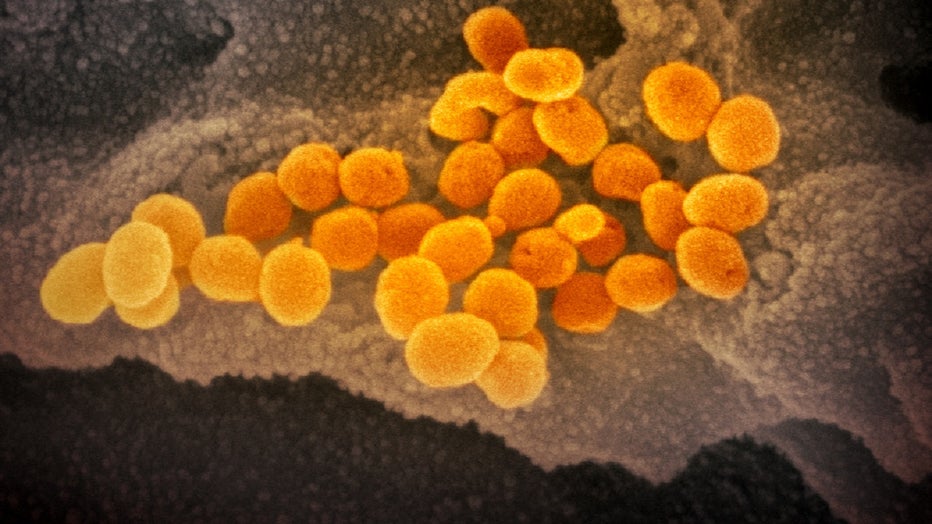
This scanning electron microscope image shows SARS-CoV-2 (orange) — also known as 2019-nCoV, the virus that causes COVID-19. (National Institute of Allergy and Infectious Diseases-Rocky Mountain Laboratories, NIH)
“Older adults are at a higher risk of suffering serious complications and needing hospitalization if they develop a COVID-19 infection,” says Evan Anderson, MD, principal investigator of the vaccine study at Emory and associate professor of medicine and pediatrics at Emory University School of Medicine and Children’s Healthcare of Atlanta. “Since many older adults don’t develop as strong an immune response to vaccines, it’s critically important for us to evaluate this vaccine candidate in older adults as well.”
Know how the COVID-19 outbreak is impacting Georgia
The vaccine, which is called mRNA-1273, was developed by NIAID and Moderna, Inc. According to Emory University, the vaccine is based on messenger RNA, which tells some cells in the body to make a viral protein and can allow for faster vaccine development compared to older methods.
Participants in the testing will receive two shots of the experimental vaccine about one month apart and will be followed by investigators for around one year. Regular safety reviews are also involved.
Officials say the vaccine does not actually contain COVID-19, so cannot cause any subjects in the testing to be infected.
For more information on the trial, click here.
RELATED: CoronavirusNOW.com, FOX launches national hub for COVID-19 news and updates.

