Emory University professor weighs in on FDA panel's Opill over-the-counter birth control decision
FDA recommends OTC access to birth control
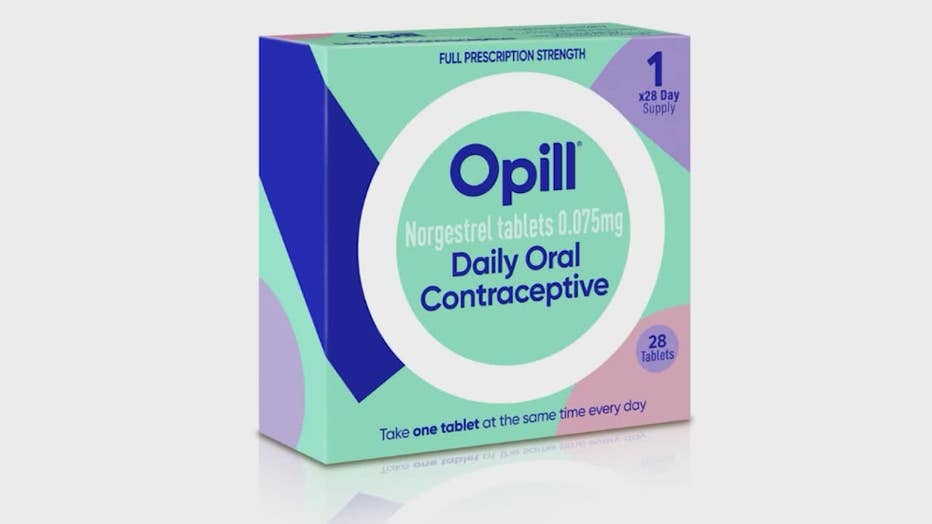
Over-the-counter birth control pills are one step closer to becoming a reality after an FDA panel recommended making the drug Opill available for purchase without a prescription.
ATLANTA - Over-the-counter birth control pills take a step closer to becoming reality. An FDA panel Wednesday recommended the drug Opill be made available without a prescription. It would be the first time consumers could buy the contraceptive medication without a doctor’s approval if the agency gives it the final okay.
Alicia Hughes, the interim executive director of the Center for Civil Rights and Social Justice and a visiting assistant professor at Emory University’s law school, called the decision "a huge tool and a huge win for women".
"This is empowering women and saying you have the right and the ability to make intelligent and informed decisions about your healthcare," Hughes continued.
Hughes, who has a background in biotechnology says Opill is safe and effective.
"It’s been approved and gone through rigorous testing from the FDA for use 6-decades ago," Hughes said.
Hughes says contraceptive pills are around available over the counter in more than 100 countries around the world. She believes the U.S. should follow suit.
"We’re actually behind the eight-ball on it. It’s revolutionary here. But it’s not something that’s revolutionary around the globe because it’s already being done," Hughes said.
Arianna Rodriguez, a student at Emory, believes birth-control pills can be an important part of women’s healthcare.
"Birth control is a very safe medication plenty of women use it," Rodriguez said.
Rodriguez supports making contraceptive pills available over the counter.
"It makes sense as a medication that would be ideal to have over the counter," Rodriguez said.
The panel’s recommendation could pave the way for full FDA approval. Hughes says the agency should carefully weigh all the evidence. The FDA is expected to make a final decision this summer.

